Touch-initiated reaction of nitrogen triiodide as a template for activation energy classroom discussions
Issue 364 | Page 41 | Published Mar 2017
Description
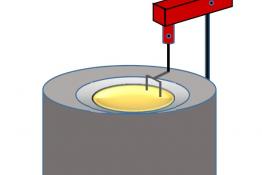
Activation energies form an energy barrier to a chemical reaction taking place. Simple collision theory, i.e. that particles need to collide to react, would suggest that activation energy is the energy needed to overcome a coulombic barrier provided by the negatively charged electrons contained within energy shells surrounding an atomic nucleus. Deriving activation energy from experiment is usually beyond the school curriculum. What can be demonstrated, however, is an almost barrierless reaction initiated by the slightest touch or vibration for the decomposition of nitrogen triiodide. This spectacular demonstration can be combined with calculations on bond enthalpies to help further understanding.
More from this issue
Increasingly affordable visualisation technology brings exciting opportunities for making the invisible appear visible. This can support the...
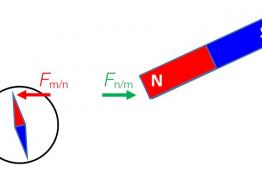
Using a conventional notation for representing forces on diagrams, students were presented with questions on the interaction between two objects....
This overview is intended to help colleagues achieve successful and satisfying observations using a ripple tank. There are many observations to...